How can healthcare researchers share their data openly and safely?
| 29 June, 2023 | Abbie nicholson |
Open data is a key component of open science practices, but it can be challenging for health researchers. From clinical trial results to patient survey responses, these researchers face many scenarios where it may seem impossible to share healthcare data openly while maintaining appropriate levels of security and privacy. However, there are ways in which this can be done – read on to find out more.
Sensitive research data in healthcare
Health research is often at the forefront of working with human participants, which leads to lots of first-hand research data.
This human data can take many forms, including:
- Blood samples, tissue samples, or genetic sequences
- Images, videos, audio files, or qualitative data related to attitudes, opinions, or experiences
- Clinical trial results
- Datasets from social media sites
- Personal identifying information, such as age, ethnicity, and sexuality
- Sensitive health status information, such as alcohol dependency
Some of these data types could allow others to identify participants if the dataset was shared openly and could lead to increased risk to participants if they were to be identified.
As a result, it’s vital that this sensitive data is handled appropriately and only shared when sufficient safeguarding measures are in place.
It’s also worth noting that other research data can be considered sensitive even if it doesn’t relate to human participants, including data that relates to:
- Intellectual property
- National security
- Third-party permissions
Some researchers may also face some of these sensitivities in their work.
Six safeguarding measures to share sensitive data openly
1. Understand ethical and legal requirements
The first step before sharing any data is to understand the relevant legal and ethical frameworks for your research.
These differ depending on where you are based, where your participants are based, or where the research is conducted, so it’s important to identify and comply with the legislation relevant to you.
In Europe, GDPR legislation governs data protection, including human data, but if participants or researchers are based elsewhere then local legislation may also apply.
Similarly, ethics must be paramount in sharing any sensitive research data, and you must ensure that you consider the rights and dignity of your participants.
Your institution, funder, or organisation can provide guidance on ethical and legal considerations for both human and non-human sensitive data. For example, the HRB provides a GDPR guide for healthcare research, and a guide to intellectual property in research.
2. Create a Data Management Plan
One of the best ways to ensure you are sharing sensitive data safely is to identify from the outset, before research even begins, the types of data you may collect or create throughout the project.
It’s also important to identify what measures must be in place to ensure you can share this data openly while maintaining appropriate security, and what type of data must not be shared at any point.
Creating a Data Management Plan and using this information will help to inform how you plan and carry out your research. In turn, this can help to identify data sharing issues in advance.
This gives adequate time to implement alternative data sharing measures, and allows you to obtain guidance from relevant institutions, ethics boards, or funders, before starting the time-sensitive publication process.
This can relate to both human data and any intellectual property or commercial data you may use or create as part of the project.
3. Gain appropriate consent
If your research involves human participants, having a Data Management Plan in place can also help to ensure you gain the appropriate consent for sharing data further down the line.
Once these consents are given, you can only share data in the way participants have consented. As a result, it’s vital you identify exactly how this will be done before starting the study to ensure you capture all situations in which you would like to share data in future.
To give informed consent, participants must have a clear understanding of how their data will be used and shared, and that they have the option to opt out or have data anonymised if they wish.
4. Anonymise data
Once you have collected the data, there are a number of methods you can use to ensure you can share data openly while maintaining confidentiality, including anonymisation.
Anonymisation removes any identifying information from a dataset to protect participant privacy and reduce the likelihood of re-identification. It’s important to note that this is not a replacement for consent and should only be done with participant data for which you have received informed consent.
Anonymising data can apply to both direct and indirect identifiers.
Direct identifiers uniquely identify a research participant and include information like:
- Full name
- Date of birth
- Address
Indirect identifiers may uniquely identify a research participant when used in combination with other identifiers, such as:
- Ethnicity
- Gender
- Sexuality
- Place of birth
- Job title
Key data anonymisation techniques include:
- Removing any variables that are not necessary for analysis or relevant to the research
- Making an information point less specific such as swapping an address for a city
- Referring to a research participant without using their real information by swapping their names for falsified versions
- Taking specific information like age and putting it into a banded range
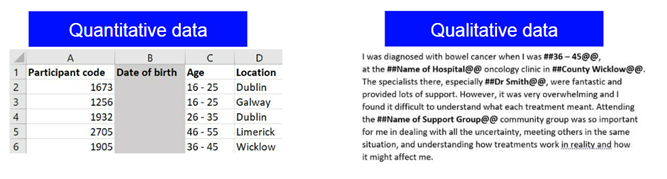
5. Control data access
In some cases, data cannot be fully anonymised, or full anonymisation would remove so much information that the dataset is no longer useful. In addition, some researchers may want to monitor and restrict access to sensitive data such as intellectual property or commercial data.
An alternative data sharing method is using a controlled access data repository (again, as long as you have obtained participant consent when sharing human data).
Controlled access data repositories provide a place in which researchers can store their data, but not publish it. Usually, a metadata record describing the data will be shared openly instead, such as a Data Availability Statement with HRB Open Research, which describes the data’s location and the conditions in which to access it.
The repository will then require that users meet certain criteria to access it, to ensure that data sharing is only done in a way that is fully controlled by researchers.
6. Publish data-related information
It’s important to recognise that not all data can be shared openly, regardless of the measures in place.
As a result, most publishers and funders will have exceptions to data-sharing policies where appropriate. For example, HRB Open Research operates an ‘open as possible, as closed as necessary’ approach, and exceptions to data sharing are outlined in the data availability policy.
However, while sharing all data may not be appropriate, authors can still publish some of their data and related information to comply with data sharing policies. This can include:
- Methods sections that provide a detailed description of how the study and subsequent data were created
- Metadata, such as Data Availability Statements, providing a description of the final data, discussion of any variables assessed, and a data sharing disclaimer
- Any intermediary data that can be shared without concern
- Detailed information about where third-party data was sourced and how users can source it themselves
This helps other researchers to reproduce the research for themselves, even if the original data is unable to be shared.
The importance of sharing data openly
Data sharing is central to open research and has many benefits for researchers, from boosting the credibility of findings to increasing the capacity for citations.
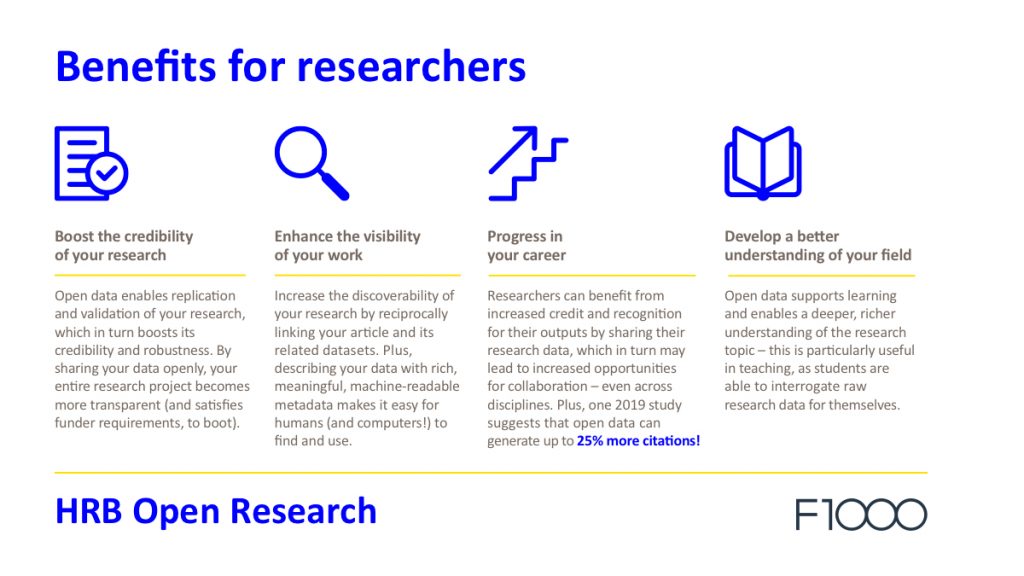
If you’d like to find out more about the benefits of sharing data, visit our Data Notes resources page.
And if you’re ready to join the thousands of HRB-funded researchers already publishing their work with HRB Open Research, submit your research for publication today.